menu
- Home
- National Information
- EC-Activities
- EUREC-Activities
- Legislation
- Training Materials
- Literature
- Events
- Newsletter
supported by:

Eurecnet - National Information: Lithuania
National Information: Lithuania
- Introduction and Historical Background
- System of Local Research Ethics Committees; Institutional Affiliation
- Legal Situation / Implementation of the DIRECTIVE 2001/20/EC
- Contact / Addresses
Introduction and Historical Overview
Since its independence Lithuania has implemented a rather comprehensive system of ethical review of biomedical research. This system is based on a two-tier model of ethical review for multicenter biomedical research and consists of a national body – the Lithuanian Bioethics Committee (LBC) and regional research ethics committees (regional RECs). This system is enforced by the Law on Ethics of Biomedical Research. Lithuania has been one of the few countries in Eastern and Central Europe where this field was regulated by a specific law.
Ethical review of biomedical research was started by two research ethics committees in the late eighties/early nineties in Lithuania. These were two institutional review boards established at Kaunas Medical University and at the Institute of Oncology in Vilnius, which were issuing approvals to conduct biomedical research projects including clinical drug trials. However, a formalized ethical review was only started after a special Decree on the Ethical Expertise of Biomedical Research of the Minister of Health has was passed in 1997. Following the Decree, the Lithuanian Bioethics Committee became the only institution authorized to issue approvals to conduct biomedical research projects (approvals for clinical drug trials had to be issued upon the recommendations of the State Medicines Control Agency (SMCA)).
A further important step in developing the system of ethical review of biomedical research projects including drug trials was the Law on Ethics of Biomedical Research, which came into force in 2001. The Law introduced a two-tier system of research ethics committees and enforced the basic principles of biomedical research ethics.
Another very important change took place in 2004 and was related to the implementation of the Directive 2001/20/EC of the European Parliament and of the Council on the Conduct of Clinical Trials on Medicinal Products for Human Use , which came into force on the11th of May, 2004. As a result, the Law on Ethics of Biomedical Research and the Law on Pharmacy were amended and harmonized with the Directive. The main change in the system was related to the shift of the roles of the LBC and the SMCA in the process of approval of clinical trials on medicinal products. Following the Directive, the role of the Competent Authority was shifted to the SMCA (since 1997 and before the implementation of the Directive, this role had been delegated to the LBC), while leaving the LBC the role of the body to issue an opinion on clinical drug trial. The right to issue approvals to conduct other types of biomedical research was left to the LBC or RBREC.
The latest amendments of the Law on Ethics of Biomedical Research came into force in 2008. These amendments have further specified the procedures of the establishment and composition of the regional RECs.
System of Local Research Ethics Committees; Institutional Affiliation
The Law on Ethics of Biomedical Research enforced the provisions for the establishment of the system, which is based on a two-tier model of ethical review for multicenter biomedical research and consists of a national body - Lithuanian Bioethics Committee (LBC) and regional biomedical research ethics committees (regional RECs).
The Lithuanian Bioethics Committee is established by and accountable to the Ministry of Health. LBC provides advice on the bioethical issues to the Ministry of Health and other governmental bodies as well as informs the general public about bioethical matters. The LBC also coordinates the activities of the regional RECs.
One of the main functions of the LBC is the ethical review of biomedical research including clinical drug trials. In order to implement this function, the Committee has established a Group of Experts of Biomedical Research. The Group is responsible for the evaluation of biomedical research projects submitted to the Committee and making decisions regarding their ethical and legal acceptability. The LBC issues approvals for those biomedical research projects which are going to be carried out in more than one region. (Regional RECs are entitled to issue approvals for the biomedical research projects that are carried out only in the region, which has been assigned to its mandate by a special Decree of the Minister of Health).
According to the Law of Ethics of Biomedical Research and the Law on Pharmacy, in order to commence a clinical trial on medicinal product, the sponsor (investigator) shall obtain a favorable opinion of the Lithuanian Bioethics Committee as well as the approval of the State Medicines Control Agency. Following the above mentioned laws, the LBC is responsible for issuing a “single opinion” for all clinical drug trials conducted in the country.
Regional RECs are based at the Universities with the tertiary medical education level. The Kaunas Regional Biomedical Research Ethics Committee was established at Kaunas Medical University in 2001. It is also planned to establish one more region committee at Vilnius University in 2008. According to the Law on Ethics of Biomedical Research, the RECs should be composed of 9 members: 4 degree-holding representatives of the respective university, 4 members nominated by the Ministry of Health and 1 member representing patients’ organization. The membership and statute should be approved by both the Rector of the University and the Minister of Health. The regional RECs are accountable to both the University and the LBC.
Researchers can appeal against the regional REC’s decision to the LBC and start the appeal procedure in the courts if they disagree with a decision of the LBC.
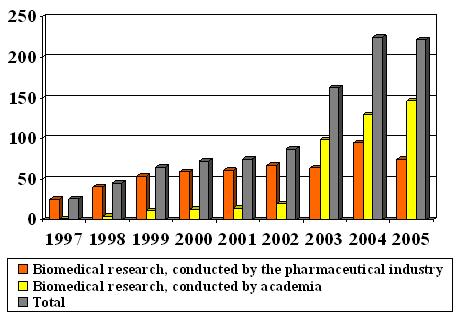
See the table of the number of new research projects, reviewed by RECs 1997-2005:
- The Directive 2001/20/EC was implemented by the Health Care Ministry Decree on the implementation of the Rules of Good Clinical Practice (GCP), which came into force on the 11th of May, 2004 (amended in 2006);
- The Convention on Humans rights and biomedicine was ratified by the Parliament of the Republic of Lithuania in 2002 and came into force on the 1st of February, 2003;
- The Additional protocol on human rights and biomedicine concerning biomedical research was signed in 2005;
- The Law on Ethics of Biomedical Research of the 11th of May, 2000, which came into force on the 1st of January, 2001;
- The Law on Pharmacy of the 22nd of June, 2006 (article 18).
- Decree on the Procedure to Issue Approvals to Conduct Biomedical Research, No. 570 (2000);
- Decree on the List of the Documents to be Presented by the Sponsor of Biomedical Research and (or) by the Principal Investigator in Order to be Authorized to Conduct Biomedical Research, No. 29 (2001);
- Decree on the Procedure for the Estimation and Covering of Expenses Incurred by Research Subjects, No. 23 (2000);
- Decree on the Rules of Compulsory Civil Liability Insurance for the Principal Investigator and the Sponsor No. 745 (2000).
Legal Situation / Implementation of the DIRECTIVE 2001/20/EC
General legal regulations
Bylaws, which regulate Biomedical research Ministry of Health:
- Decree on the Requirements for the Form and Content of documents to be presented by the Sponsor of medical research and (or) by the Principal investigator in order to be authorized to Conduct Biomedical Trial No. 09-22 (2001);
- Decree on the List of Documents to be Presented by the Sponsor of Medical Research and (or) by the Principal Investigator in order to be Authorized to Conduct Biomedical Trial No. V-21 (2004).
- Ministry of Health Decree on the Procedure to Issue Approvals to Conduct Clinical Trial on Medicinal Product, No. V-435 (2006);
- Lithuanian Bioethics Committee Decree on the Regulation for the Submission of the Documents to the Lithuanian Bioethics Committee to Issue Favourable Opinion to Conduct a Clinical Trial on Medicinal products No. V-11 (2004).
- Detailed Guidance for the Request for Authorisation of a Clinical Trial on a Medicinal Product for Human Use to the Competent Authorities, Notification of Substantial Amendments and Declaration of the End of the Trial (2006);
- Decree on Paediatric Clinical Trials No. 70 (2002);
- Decree on the Requirements for Reporting Serious Adverse Events (SAEs) and Serious Adverse Drug Reactions (ADRs) from Clinical Trials (CTs) No.: 1A-633 (2006).
Legal Situation / Implementation of the DIRECTIVE 2001/20/EC
Lithuanian Bioethics Committee:
Bylaws, which regulate clinical trials on medicinal products:
State Medicines Control Agency of Lithuania (SMCA):
Contact / Addresses
Lithuanian Bioethics Committee
Didzioji 22, Vilnius
tel./fax. +370 5 2124565
e-mail: lbek@sam.lt
website: http://bioetika.sam.lt
<- National Information